Image-based computational fluid dynamics
|
|
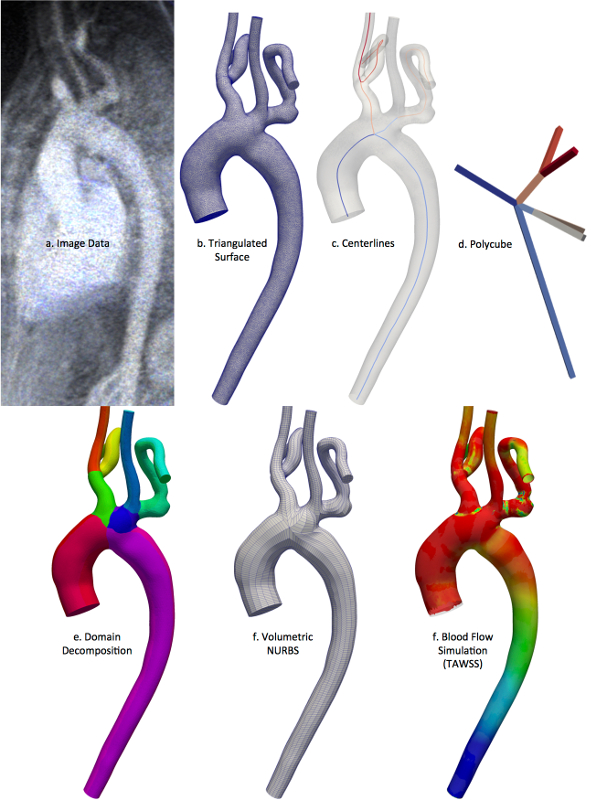
Current state of the art in image-based vascular hemodynamics modeling involves construction of a personalized computer model that serves as the computational domain for fluid dynamics simulation of blood flow. Geometry is derived from medical image data such as CT or MR using local or global image processing techniques. The resulting model represents a truncated section of a nominally closed-loop vascular system. Therefore proper inflow and outflow conditions, or reduced order models, must be imposed at the inlets and outlets, and under appropriate conditions the fluid mechanics must be coupled to the vascular dynamics.
|
Lagrangian coherent structures
|
|
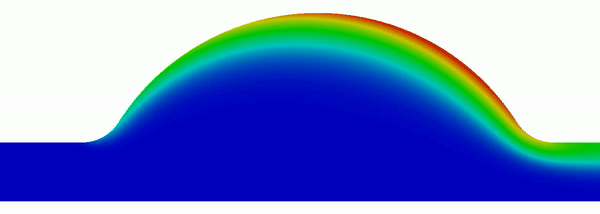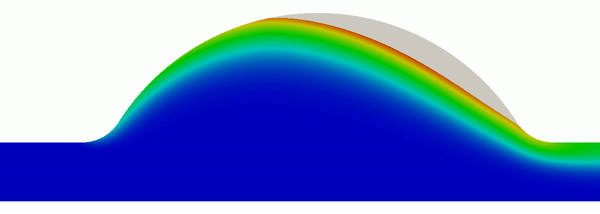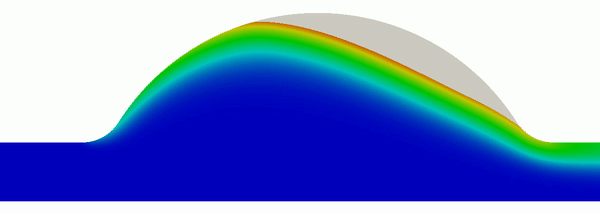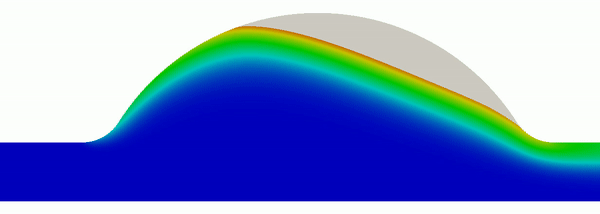
The computation of Lagrangian coherent structures (LCS) enables a systematic approach to precisely capture fundamental boundaries in dynamical systems, whose coordinated dynamics provide the template for chaotic transport. In essence, these structures often generalize, or are analogous to, the computation of stable and unstable manifolds of hyperbolic fixed points to aperiodic, finite-time systems where fixed points no longer exist. We work to develop mathematical and computational advancements of this framework, in addition to utilizing this methodology to better understand a wide range of real-world problems. An informative, but outdated, tutorial can be found here.
|
Abdominal aortic aneurysm disease
|
|
Biomechanical factors play an important role in the localization and progression of abdominal aortic aneurysm (AAA). Blood flow in the thoracic and descending aorta is mostly unidirectional, whereas infrarenal aortic flow is characterized by recirculation and low and oscillatory wall shear stress. This results in a 10-fold increase in risk for aneurysm formation in the infrarenal versus suprarenal aorta. Once formed, AAA worsens hemodynamic conditions, further perpetuating disease progression. The exact biomechanical mechanisms underlying AAA pathology are not well understood. We seek to integrate advances in medical imaging, flow modeling and biomechanics analysis to better understand the relationships between AAA biomechanics and disease progression. In collaboration with Dalman Research Lab at Stanford.
|
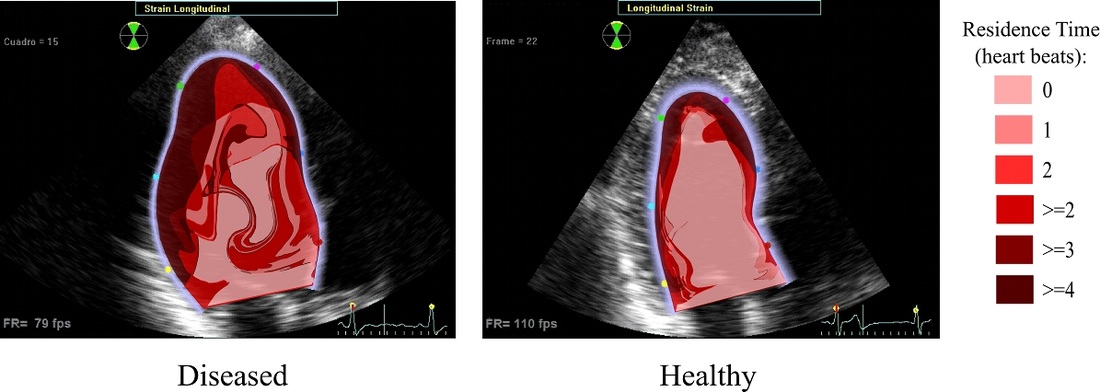
Intraventricular hemodynamics
|
Heart disease can lead to impaired ventricle function resulting in decreased blood ejection and increased blood stasis inside the heart. While ejection can be measured clinically, stasis and transport quantification can be more elusive. Novel processing of color-Doppler echocardiography can be used to reconstruct the flow field inside the left ventricle. This flow data can then be coupled with computational techniques to uncover blood injection, ejection and transport patterns and mixing. Such information is needed for diagnostic purposes but also in treatment planning, especially cardiac resynchronization therapy. This project in collaboration with JC del Alamo at UC San Diego.
|
Particle-Continuum Computational Approaches To Investigate Thrombosis And Embolisms
|
Pathological blood clotting (thrombosis) and flow-disruption by fragmented clots (embolism) are crucial vascular events that comprise the leading cause of multiple diseases including heart attack and stroke. Comprehensive understanding of these disease phenomena in patients is often precluded by the complexity of the underlying multiscale biomechanical processes, especially in large arteries. Our research focuses on probing into this complexity using medical image-driven multiscale particle-continuum computational approaches. The methods developed, and subsequent findings from numerical simulations, have: (a) revealed the role of pulsatile large-artery flow in distributing emboli (fragmented clots) to various vascular beds; and (b) enabled realistic predictions of flow and transport around large arterial clots with arbitrary shape and morphology. This work was supported by the American Heart Association, National Science Foundation, and the Burroughs Wellcome Fund.
|
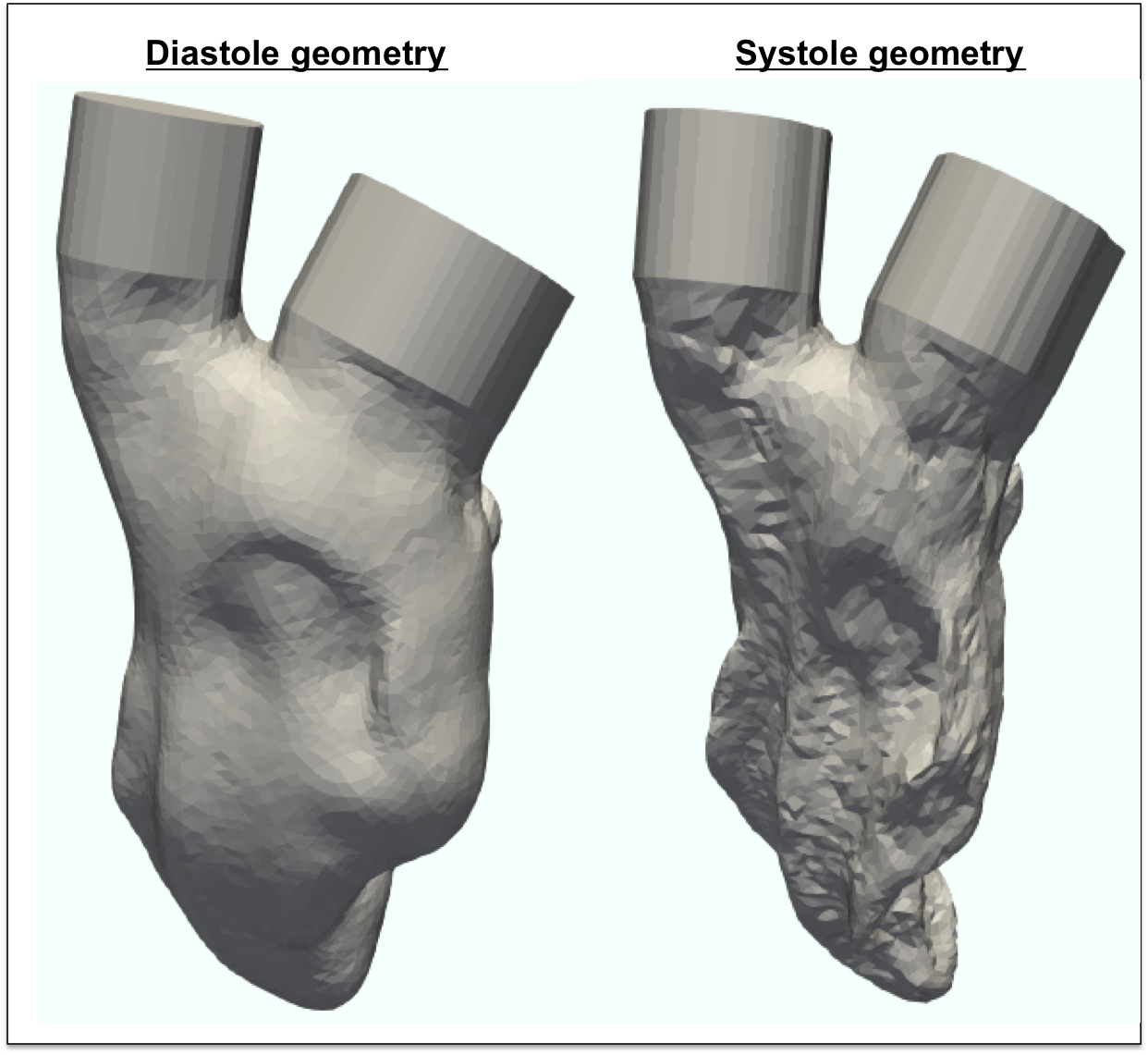
Fluid-Structure Interaction Simulation of the Left Ventricle
|
An abnormal flow pattern in the left ventricle may indicate the development of heart diseases and may contribute to the progression of heart failure. Clinical measurements can provide ejection fraction, cardiac output and stroke volume, but cannot obtain the full hemodynamics information within the left ventricle. Fluid-structure interaction method couples the dynamics of left ventricle tissue and blood flow to capture the contraction of the left ventricle and the left ventricle hemodynamics. Such method can be applied on patient-specific simulations to visualize the intraventricuar flow and improve the early prognosis of heart diseases.
|
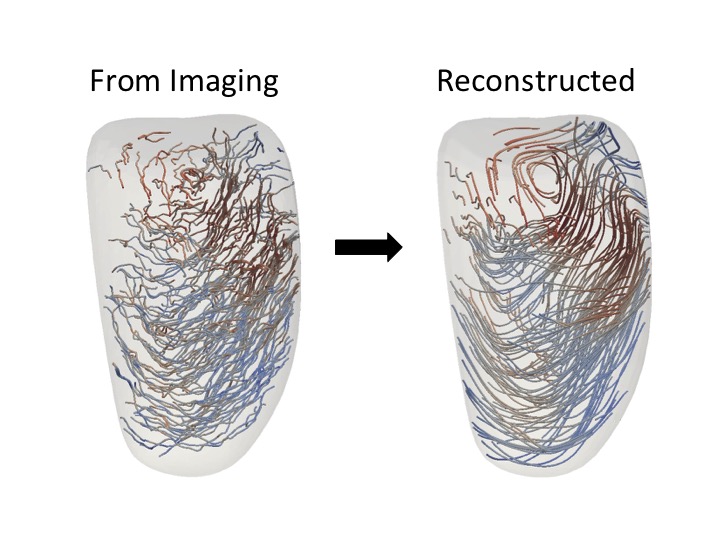
Analysis Suitable Vascular Model Construction
|
SimVascular (http://www.simvascular.org) is a completely open source pipeline for image-based blood flow modeling and simulation that is used all over the world for investigation of cardiovascular diseases. It is very important that the tools in SimVascular are effective, accurate, and easy to use. Of particular importance is the modeling of arteries from image data. This is typically one of the most time-intensive steps, and it is also one of the most critical. Slight altercations in arterial geometries have profound effects on the results. This research is geared towards creating and developing open source tools to aid in arterial modeling. Recently, advanced modeling techniques to represent complex arterial models as the industry computer aided design (CAD) standard have been investigated. These methods are being created in a unique way so that the final model representations can also be used directly for analysis using an increasingly popular finite element analysis (FEA) alternative called isogeometric analysis (IGA).
|
Reduced-order modeling of thrombosis
|
Computational modeling of large artery thrombosis is challenging for several reasons. First, it involves a wide range of spatial and temporal scales. Blood flow dynamics span up to centimeters, while platelet interactions and near-wall chemical concentration boundary layers are on the order of microns. Second, the underlying processes are highly complex and in some cases not fully understood. Simulation of the coagulation cascade alone, for example, requires the knowledge of dozens of reaction rates and initial conditions, many of which are not well known. We aim to develop reduced-order models that help span these spatial and temporal scale discrepencies as well as reduce model complexity, with the goal of simulating thrombotic processes in three-dimensional, patient-specific arterial geometries.
|
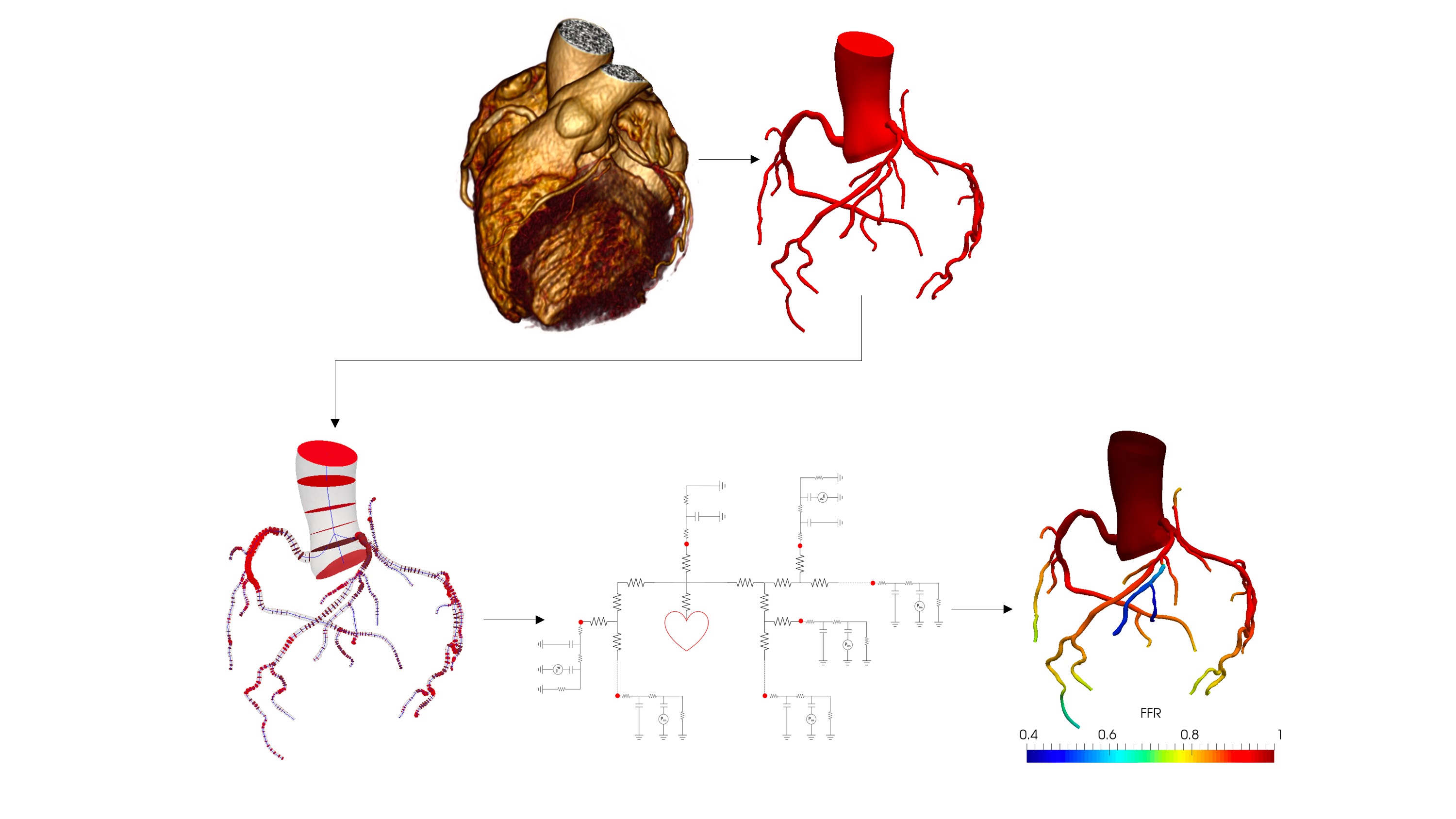
Coronary flow simulation
|
Coronary artery disease is a leading cause of death worldwide. It manifests when the coronary arteries that supply the oxygen-rich blood to myocardium and heart muscle become abnormally narrowed with material known as plaque. Excessive plaque build-up and its rupture can obstruct blood flow and result in chest pain, ischemia or heart attack. Fractional flow reserve (FFR) which is the ratio of the mean blood pressure downstream of the plaque to the mean aortic root pressure, is considered the gold standard to determine the functional significance of a coronary artery stenosis. FFR requires invasive measurement, by inserting a special pressure probe through the aorta, thus there is nontrivial risk and cost associated with this diagnostic test. We are working on developing an automated physics-based framework for non-invasive real-time assessment of FFR based on geometric features extracted from medical image data.
|
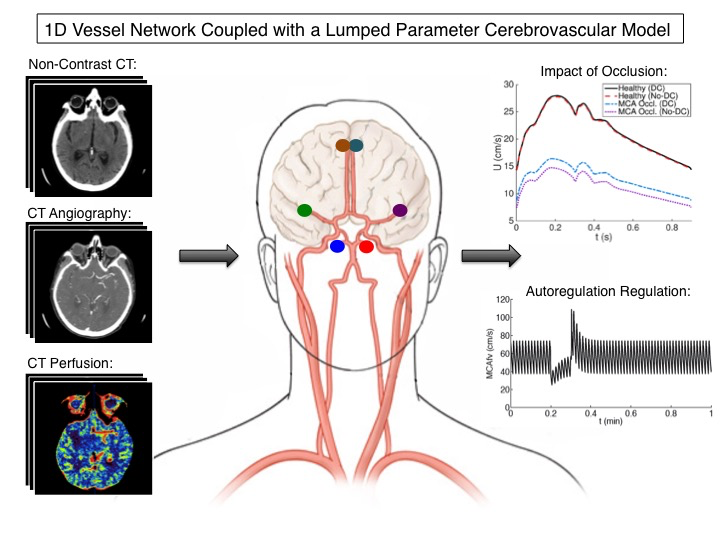
Reduced-Order Cerebrovascular Modeling of Ischemic Stroke
|
Each year, over 700,000 devastating incidences of stroke occur in the United States alone. Upon arrival to the hospital, each stroke patient undergoes a series of CT/MRI scans so that doctors can diagnose the stroke type/severity and then decide which treatment plan the patient will best respond to. However, even with these scans, determining the best treatment is still a difficult decision due to the complex cerebral hemodynamics of each unique individual. An individual’s brain blood flow is determined by the interconnected mechanisms of cerebral autoregulation, cortical collateral vessels, communicating arteries, cerebrospinal fluid, and intracranial pressure dynamics — all of which influence a patient’s response to an ischemic stroke and subsequent treatment. To better understand this complex system and provide blood flow predictions on a patient-specific basis, reduced-order cerebral hemodynamic computational models are being designed to model a patient’s baseline healthy and ischemic stroke hemodynamic states to eventually predict their post-treatment hemodynamic response. Specifically, medical image data from an ischemic stroke patient such as non-contrast CT, CT angiography, and CT perfusion are all being used to tune these computational models for a particular patient. The logic here is that a properly tuned computational model can better predict a patient’s post-treatment blood flow by taking their complex and unique cerebral hemodynamics into account thus providing doctors with a complementary tool to improve understanding of treatment efficacy and risk.
|
Flow Image Post-Processing
|
Blood flow patterns in the left ventricle have been correlated with heart disease, but our current understanding of how blood flow contributes to disease progression is incredibly limited. This lack of understanding is largely due to our limited ability to image blood flow. MRI is the current gold standard, but it is expensive and unavailable to patients with metal implants such as pacemakers. Color-Doppler ultrasound is an inexpensive, easy-to-use tool, but the velocity information is incredibly limited. We are developing processing techniques for flow image data that can be used to calculate a fully 3D flow field in the left ventricle, enabling studies for improved understanding of how flow contributes to heart disease, and ultimately leading to improved diagnosis and treatment.
|
High-resolution In-silico Models of Total Heart Function
|
Biophysically detailed computational (in-silico) models of human heart function integrate methods from different disciplines: electrophysiology, tissue mechanics, hemodynamics, metabolism, cell mechanics & biology. It is characterized by a large scope of both clinical and industrial applications, a wide variety of data sources used (imaging, sensors, omics), and its focus on multiple biological scales (organ, tissue, cell). To simulate cardiovascular processes in a given patient, parameterization and data assimilation techniques are developed to replicate measurable clinical metrics. From a clinical perspective computational models bear high promise as a cardiovascular diagnostic modality. Simulation results aid in better understanding disease processes and improve patient evaluation and treatment. Optimizing cardiac devices such as pacemakers and artificial heart valves are other important applications. Moreover, in-silico analyses offer the unique opportunity to predict quantities in patients which cannot be measured in vivo such as tissue and wall shear stresses, that can be essential to identify regions prone to atherosclerosis, infarction, or aneurism development. Thus, research into advancing methodology for in-silico clinical applications are poised to have a significant impact on future health care. |
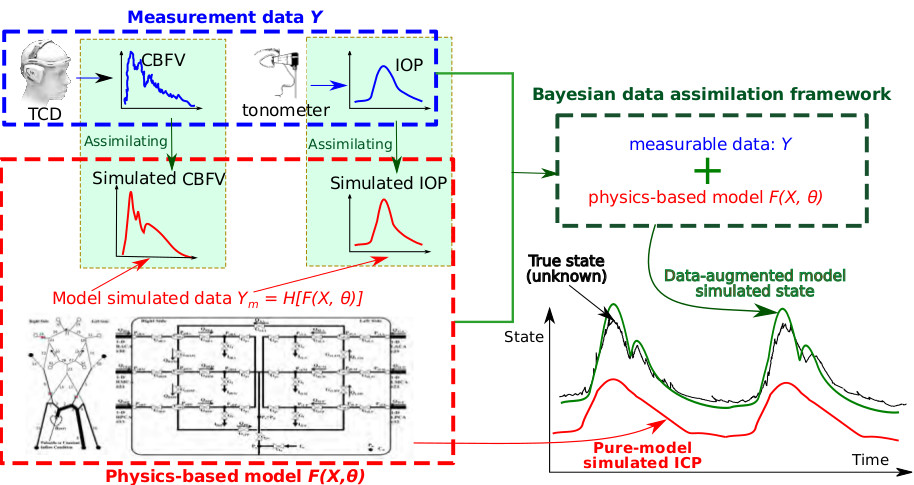
Data assimilation for cardiovascular modeling
|
Intersubject variability requires theory-based computational models of cardiovascular function to be as individualized as possible. Medical imaging has largely provided the capability to create “subject-specific” models. In addition to the traditional use of medical image data for specifying model geometry and boundary conditions, we further explore using the data to identify model parameters and improve hidden states estimation. This is done using data assimilation and inverse modeling techniques, which combine measurement data with computational models in a way that minimizes the discrepancy between the two. Specifically, we are developing data assimilation methods for noninvasive intracranial pressure (nICP) estimation and solving inverse problems for bio-related solid mechanics applications.
Researchers involved in this projects: Dr. Jian-Xun Wang, Miguel Rodriguez. |
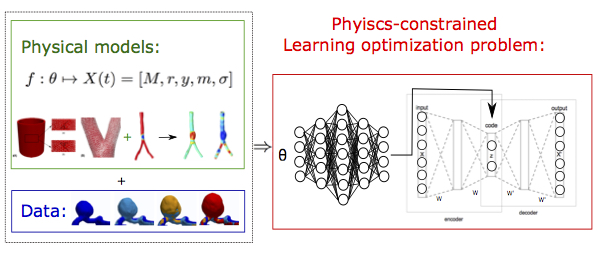
Physics-informed machine learning in biomedical applications
|
Due to the advancement of sensor technology and computation powers, data-driven machine learning approaches have been widely used and proved to be competent in a number of of real-life prediction problems. However, for most biomedical applications, the training data are commonly insufficient. On the other hand, associated physiological knowledge and underlying physics have been continuously advanced. Therefore, in this project, we are developing physics-constrained machine learning techniques, which imposed prior known knowledge and physical laws to achieve efficient training and robust predictions, in the circumstance of lack of enough data and need of interpretability. Specifically, we are investigating physics-based constrained optimization and gradient sampling methods in the statistical learning process for biomedical applications.
Researchers involved in this project: Jiacheng Wu, Dr. Jian-Xun Wang. |